AMNOG-Dossier
Market launch and early benefit assessment of a medicinal product in Germany
The German Act on the Reform of the Market for Medicinal Products (AMNOG) came into force in 2011. Since then, if you want to place a medicinal product on the German market, you must submit an extensive dossier to the German Federal Joint Committee (G-BA). The dossier demonstrates the added benefit of a new product compared to an appropriate comparator treatment, which is determined by the G-BA.
The AMNOG dossier is created in accordance with detailed specifications from the G-BA. In a benefit assessment, the documents for non-orphan drugs are reviewed by the Institute for Quality and Efficiency in Health Care (IQWiG). Dossiers for orphan drugs are usually evaluated by the G-BA. The German National Association of Statutory Health Insurance Funds (GKV-Spitzenverband) uses the AMNOG dossier and the benefit assessments as a basis for pricing.
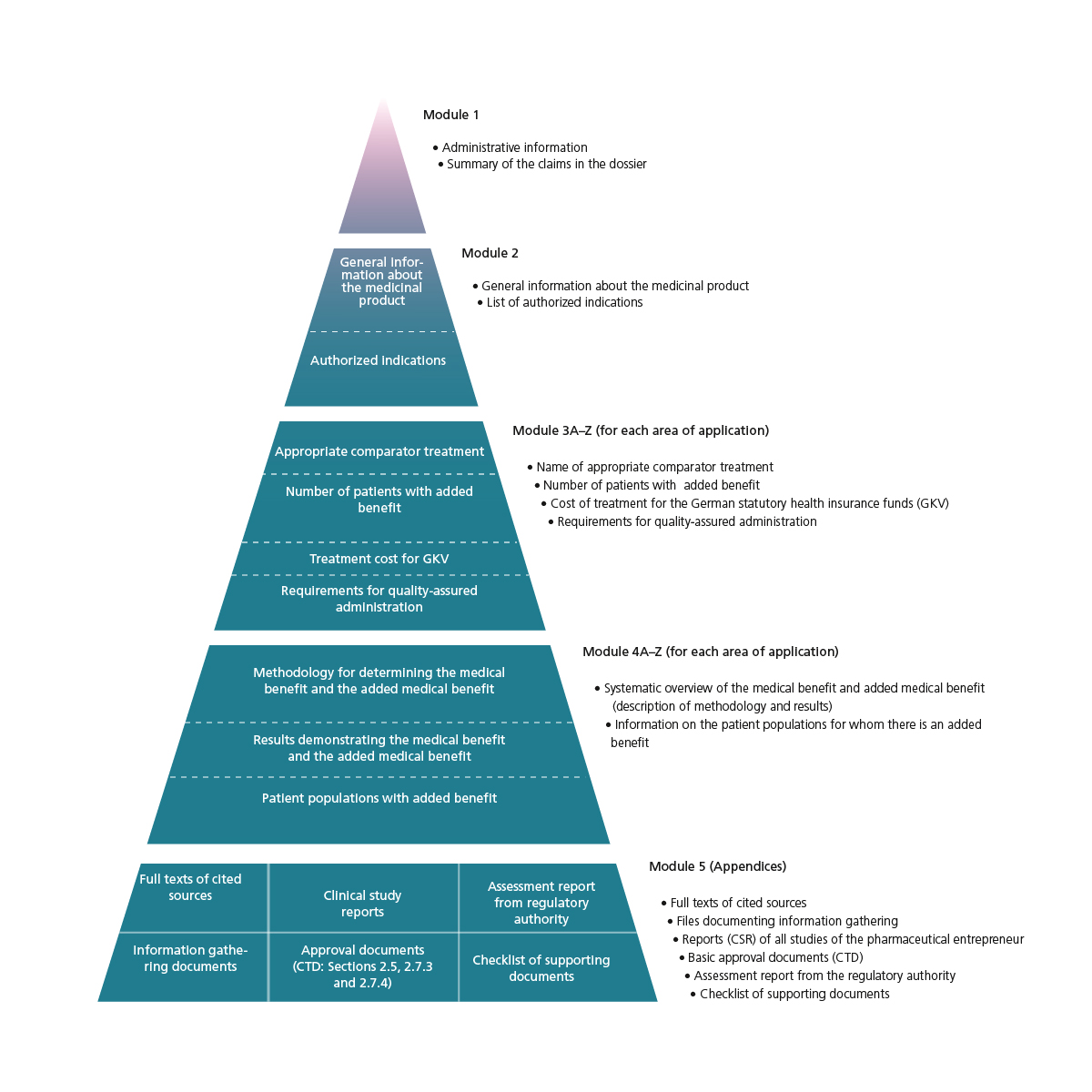
The five modules of the AMNOG dossier
- Module 1 of the dossier summarizes the content of modules 2 to 4.
- Module 2 describes the medicinal product and lists the approved areas of application.
- Module 3 specifies the appropriate comparator treatment, the number of patients in the authorised indication and the treatment costs for German statutory health insurance providers (GKV). It also describes the disease, epidemiology, unmet need and requirements for quality-assured administration. Furthermore, the module contains information on how to adjust the Uniform Value Scale (EBM) if required.
- Module 4 analyses the extent of the added medical benefit and the validity of the evidence.
- Module 5 contains documents that support the claims made in modules 2 to 4, plus a checklist for ensuring that the dossier contains all necessary documents.
What does an AMNOG dossier look like?
At the heart of the benefit dossier is a detailed statistical presentation, in particular of randomized studies, comparing the benefit of the new medicinal product with that of the appropriate comparator treatment. In nearly half of all cases, two or more patient populations need to be differentiated and analysed separately. These populations will often require different comparator treatments.
The focus of the analyses is on patient-relevant outcomes in the areas of mortality, morbidity, safety and quality of life. Patient-reported outcomes (PRO) play an important role here. Similar to adverse events, they must be presented in a level of detail that usually exceeds that of the clinical study reports. In addition, comprehensive subgroup analyses must be performed for all outcomes. For international studies these should include proof of the transferability of the results to Germany.
The AMNOG procedure step by step
As well as compiling the AMNOG dossier, the Institute for Evidence-Based Positioning in the Healthcare Sector offers other services related to the AMNOG procedure. These include strategic consulting from the perspective of the payer and preparation for hearings and negotiations.
Services in detail
AMNOG dossier creation – benefiting from many years of experience
Professor Dietrich was for many years a deputy member of the decision-making body of the German Federal Joint Committee (G-BA) and was responsible for controlling national pharmaceutical expenditure at the German National Association of Statutory Health Insurance Physicians (KBV).
In the course of her consulting activities she has created a great number of AMNOG dossiers and has supported and advised pharmaceutical companies in hundreds of projects.
Her experience covers ophthalmology, infectious diseases, oncology, dermatology; respiratory, cardiovascular and metabolic diseases; disorders of the musculoskeletal, urogenital, digestive and nervous systems; and haematological diseases.